Abstract
Introduction:
In the past decade, targeted inhibition of BCR-ABL1 with tyrosine kinase inhibitor therapies (TKIs) has become the standard-of care for treatment of chronic myeloid leukemia (CML). Second-generation TKIs, including dasatinib and nilotinib, have been shown to be more effective treatment options over imatinib and are approved as first-line therapies for newly diagnosed patients (pts) with Philadelphia chromosome positive CML in chronic phase (CP) and as second-line therapies for pts in CP and accelerated phase resistant to or intolerant to prior therapy. Although TKIs have been demonstrated to prolong overall and progression-free survival, there is evidence that TKIs may be associated with an increased risk of infections. This risk of infections can occur with or without neutropenia and is potentially due to an immunosuppressive effect from off-target activity of the treatment (Reinwald 2016). The real-world healthcare resource utilization (HRU) and costs related to infections have not been well studied, particularly in pts diagnosed with CML treated with second generation TKIs. To address this gap, this study compared HRU and costs of pts receiving dasatinib or nilotinib in first-line therapy for CML, with a focus on infection-related economic outcomes.
Methods:
Adult pts newly diagnosed with CML who were initiated on dasatinib or nilotinib as first-line therapy on/after October 2010 were identified from 2 large US administrative claims databases (2006-2016). First-line TKI initiation was defined as the index date, the 6-month period before the index date was defined as the baseline period, and pts were observed from index date until the end of first-line TKI therapy (i.e., discontinuation, switch, end of data availability/continuous health plan enrollment). HRU, including inpatient (IP) days, emergency room (ER) visits, and days with outpatient (OP) services, and associated healthcare costs from a payers' perspective (USD 2016) were measured during first-line TKI therapy. All-cause HRU and costs and those related to infections of any type, identified using diagnosis recorded in medical claims, were compared between dasatinib and nilotinib pts using multivariate regression models.
Results:
A total of 1,156 dasatinib and 677 nilotinib pts met the sample selection criteria. Mean age was 52 years and 54% were male in both cohorts (p>.05). Mean TKI therapy duration was 13.6 months for dasatinib and 15.5 months for nilotinib (p=.014).
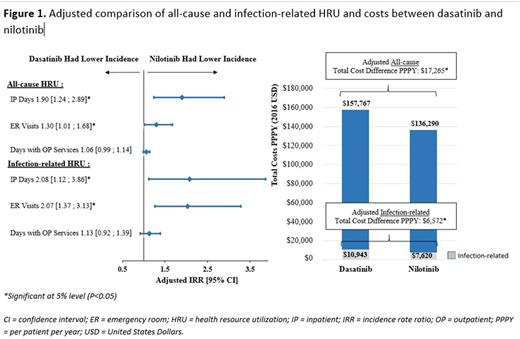
On average, dasatinib pts had 2.7 all-cause IP days annually, where 1.4 (52%) were related to infections; nilotinib pts had 1.3 all-cause IP days annually, where 0.6 (46%) were related to infections. Dasatinib pts had 90% more all-cause IP days (adjusted IRR=1.9; p=.003) and 2 times more IP days related to infections (adjusted IRR=2.1; p=.020) than nilotinib pts (Figure 1). On average, dasatinib pts had 0.7 all-cause ER visits annually, where 0.2 (29%) were related to infections; nilotinib pts had 0.6 all-cause ER visits annually where 0.1 (16%) were related to infections. Dasatinib pts had 30% more all-cause ER visits (adjusted IRR=1.3; p=.041); 2 times more ER visits related to infections (adjusted IRR=2.1; p<.001) than nilotinib pts (Figure 1). On average, dasatinib pts had 27.5 days with all-cause OP services annually, where 1.6 (6%) were related to infections; nilotinib pts had 26.0 all-cause days with OP services annually where 1.5 (6%) was related to infections (difference not statistically significant).
On average, the annual all-cause total cost was $157,767 for dasatinib pts with $37,110 (24%) for medical service costs and $120,657 (76%) for pharmacy costs. Of this total cost, $10,943 (7%) was related to infections. On average, the annual all-cause total cost was $136,290 for nilotinib pts with $24,508 (18%) for medical service costs and $111,782 (82%) for pharmacy costs. Of this total cost, $7,620 (6%) was related to infections. Dasatinib pts incurred higher all-cause total costs by $17,265 per patient per year compared to nilotinib pts (adjusted cost difference; p=.004); where $6,572 (38%) of the difference was related to infections (adjusted cost difference; p=.004; Figure 1). Overall, among pts with infection, the average cost related to infection during first-line TKI therapy was $9,637.
Conclusion:
Dasatinib was associated with higher HRU and healthcare costs, particularly related to infections, compared to nilotinib.
Seiter: Novartis: Consultancy, Honoraria, Speakers Bureau; Incyte: Consultancy, Honoraria, Speakers Bureau; Celgene: Honoraria, Research Funding, Speakers Bureau. Latremouille-Viau: Analysis Group, Inc.: Employment; Novartis: Other: Analysis Group, Inc. has received consulting fees from Novartis. Guerin: Novartis: Other: I am an employee of Analysis Group, which received consulting fees from Novartis; AbbVie Inc.: Other: I am an employee of Analysis Group, which received consulting fees from AbbVie ; Analysis Group, Inc: Employment. Ndife: Novartis Pharmaceuticals Corporation: Employment. Habucky: Novartis: Employment. Joseph: Novartis Pharmaceuticals Corporation: Employment; Pfizer: Other: stock/stock options; Amgen: Other: stock/stock options; Express Scripts: Other: stock/stock options. Pivneva: Analysis Group, Inc: Employment; Novartis: Other: Analysis Group, Inc. has received consulting fees from Novartis. Gagnon-Sanschagrin: Novartis: Other: Analysis Group, Inc. has received consulting fees from Novartis; Analysis Group, Inc.: Employment. Tang: Novartis: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal